Ruirui Sun, Paul Ranjard, 31 July 2025, first published by WTR
- Glaxo Group registered the mark DERMOVATE in Class 5 in 1988
- When Glaxo Group refiled the mark and expanded the list of goods in Class 5, the CNIPA refused the application based on Article 10.1.7
- Following Glaxo Group’s request for review, the CNIPA overturned the refusal
Current practice
This case illustrates the evolving trademark practice of the China National Intellectual Property Administration (CNIPA), which has been applying a stricter interpretation of Article 10.1.7 of the Trademark Law (deceptiveness). The CNIPA’s Trademark Examination and Trial Guidelines (2021) prescribe as follows:
- Deceptive signs refer to those that inaccurately represent the quality, characteristics or origin of the designated goods or services, thereby likely causing the public to form a mistaken understanding of such attributes or source.
- The deceptiveness assessment of a sign must be made on a case-by-case basis, taking into account the specific characteristics of the designated goods or services.
This practice has led to frequent refusals of trademark applications and, consequently, frequent requests for review of these decisions. The proportion of such review decisions in relation to all review decisions by the CNIPA almost doubled from 5.4% in 2019 to 10% in 2024:
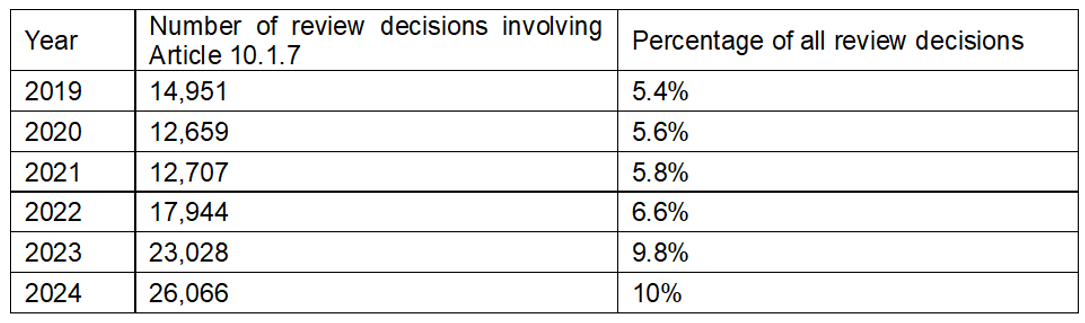
Source: MOZLEN
This situation may even occur when an already registered trademark is re-filed with an expanded list of designated goods.
Background of the case
This is precisely what happened to GSK, one of the oldest and largest pharmaceutical companies in the world. In 1988 Glaxo Group Limited, which is part of the GSK Group, registered the trademark DERMOVATE in Class 5 (“pharmaceutical preparations for the treatment and/or remission of skin diseases”).
On 21 December 2023 – 35 years later – Glaxo Group refiled the trademark in Class 5, expanding the list of goods by adding, among others, “pharmaceutical preparations; skin care pharmaceutical preparations”.
This time, the application was refused by the CNIPA, citing Article 10.1.7 of the Trademark Law. The CNIPA held that the term ‘derm’ could be interpreted as referring to ‘dermis’ or ‘skin’, thus making the trademark misleading regarding the functions and usage of the designated goods.
Glaxo Group filed an application for review of the refusal, presenting the following arguments:
- The term ‘derm’ does not constitute an independently recognisable component in the applied-for mark, and it is unlikely that consumers would isolate and interpret it separately. Further, the mark DERMOVATE, as a whole, is not commonly used or understood by the general Chinese public. It is thus inherently distinctive and does not convey any specific meaning that could mislead consumers as to the characteristics of the goods.
- The mark DERMOVATE has been successfully registered in Classes 3 and 5 in China and various other jurisdictions. The CNIPA should follow consistent examination standards in assessing this new application.
- The mark DERMOVATE has been used by GSK for many years. In English-speaking jurisdictions, no deceptiveness issues have arisen. It would be unreasonable to assume that Chinese consumers, who may have limited familiarity with English, would be misled solely due to the presence of the term ‘derm’ in the mark.
In support of the review request, Glaxo Group submitted
- dictionary search results for the term ‘dermovate’;
- registration records of other trademarks containing ‘derm’;
- precedents where the CNIPA or the courts overturned refusals based on deceptiveness; and
- evidence demonstrating the global use and registration history of DERMOVATE by Glaxo Group.
Decision
On 10 April 2025 CNIPA concluded that there was insufficient evidence suggesting that the trademark would likely mislead the relevant public regarding the functions or characteristics of the designated goods. Therefore, the mark was approved for registration.
Comment
Against the backdrop of increasingly rigorous examination criteria and frequent citation of absolute grounds for refusal, the result is quite satisfactory.
Although Glaxo Group secured a partial win in reversing the unreasonable refusal, the success rate of overcoming refusals based on deceptiveness remains relatively low; it is believed to be hovering around 10.2% in 2024 according to a third-party database.
It is therefore recommended that brand owners, when re-filing an already registered trademark, conduct a risk assessment analysis. Given that Article 52 provides for a fine of up to 20% of the turnover in cases of violation of Article 10, the risk caused by the current examination practice could be high.